
SealSac Embolic Coil System Granted NMPA Registration Certificate
2022-03-16 19:32:23

In Mar, 2022, Shanghai HeartCare Medical Technology Corporation Limited received approval from the National Medical Products Administration for SealSac Embolic Coil System, which is the 11th product ever launched and marks another milestone towards the target to provide one-stop solution and become the industry leader for stroke therapeutics and prevention, with our full-set pipeline of products for acute ischemic stroke ,neurovascular stenosis treatment, ischemic stroke prevention, hemorrhagic stroke treatment, and interventional vascular access.
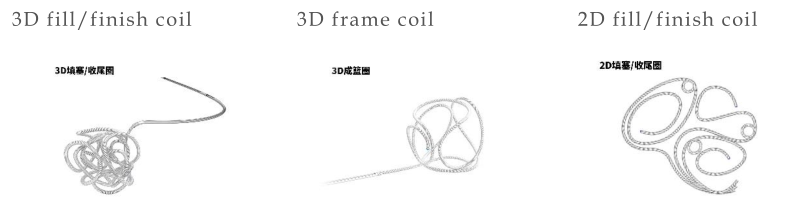
Features for SealSac Mechanical Detachable Coil
Stable
▪Stable delivery and immediate detachment without kick back
▪Good endothelialization and bio-compatability for high cure rate due to multi-dimension design of frame coil
Secure and Smooth
▪Secure framing across aneurysm neck, dense volume filling and soft finishing coils
▪The coil can safely enters into the aneurysm and it can flexibly seek out open spaces to fill in more volume due to smaller outer diameter of tip for 2nd configuration coil
▪Suitable for frame, fill and finish for giant, large, medical to small and tiny aneurysm with various models